Why does the charging time of old batteries (lower capacity) not become shorter? | Battery Monday
Why does the charging time of old batteries (lower capacity) not become shorter? | Battery Monday
Nowadays, it’s common knowledge that batteries lose their ability to retain a charge as they age. But why do they still take as long, if not longer, to charge than before? As Li-ion batteries get older, the charging time does not drop even if the accepted capacity is no longer 100%. This is called "capacity loss or fading", which is what we’ll discuss in today’s Battery Monday.
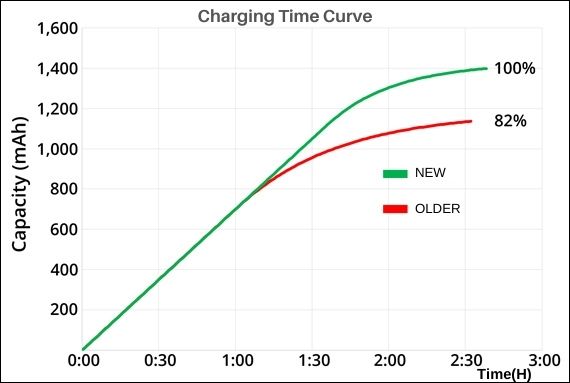
Comparison of old and new battery charging
The graph here shows the charging time of a new lithium-ion battery with a capacity of 1,400 mAh (100% SOC) and an older battery pack providing only 1,150 mAh (82% SOC), both of which take approximately 150 minutes to recharge at 0.5C.
The next graph shows the different charge saturation times for the old and new batteries. The batteries are charging at a constant current of 700mA, then at a constant voltage of about 0.05C to finish. The data for the new battery, shown in green, starts curving later, and you can see how it has a short trailing edge, while the old battery, shown in red, has a longer trailing edge.
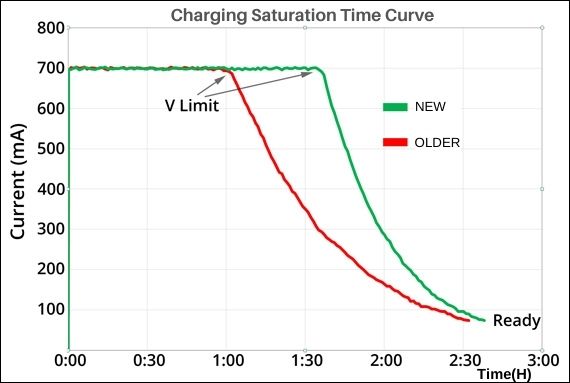
This edge indicates that old lithium-ion batteries' charging time will not be shortened due to lower capacity. To use an analogy, a young athlete has little or no deceleration at the end of a sprint, while an older person is out of breath and starts walking, thus extending the time to reach the goal.
A common aging effect
A common aging effect of Li-ion is the loss of electrical charge transfer capability due to a bad chemical reaction. In a healthy cell, ions flow freely between the cathode and anode. Charging a battery forces ions to flow from the negative electrode to the positive electrode, and discharging them reverses the flow. Theoretically, it should work forever as a permanent energy machine. But this is not the case. The amount of battery wear and tear is directly related to the frequency of battery use.The loss of charge in a battery starts at the electrodes, usually containing nickel in their complex composition. Since we cannot create a completely smooth surface inside the battery, there will always be small nooks, crannies, and gaps. Thus, as the ions pass through the cell's positive and negative terminals, some of them come into touch with the nickel, causing a reaction and getting stuck, producing crystalline formations known as dendrites.The more charge and discharge cycles, the more crystals are formed, and the greater the loss of efficiency and capacity, which has the unfortunate effect of causing the battery to lose charge. High-end lithium polymer batteriesmay lose about 20% of their capacity after 1000 charge cycles, such as NMC811 batteries.Just like with human lives, aging is permanent and cannot be reversed. So when using your battery, ensure you follow your manufacturer’s instructions and educate yourself on how to maintain the life of your electronics.
Video
Link: https://www.youtube.com/watch?v=r-JZreNWwGc
Grepow's Battery Monday Channel is about battery knowledge and some battery tips. If you have any questions about this topic (Why does the charging time of old batteries not become shorter? ) or have any battery-related things you want to know, please feel free to contact us by email at info@grepow.com. We specialize in drone smart batteries, industrial batteries, shaped batteries, high discharge rate batteries, high voltage batteries, etc. Or if anything we could improve, email us, we will read the comment and provide a high-quality battery knowledge tutorial.
Grepow official website: https://www.grepow.com/
Grepow Facebook: https://www.facebook.com/grepowbattery
Grepow Linkedin: Grepow Battery Linkedin
Related Articles
-

Powering Drone Soccer: High-Performance Batteries Take Flight in Singapore
2025-07-23 -

LiPo battery vs LiHV battery: what’s the difference?
2025-07-16 -

Building an FPV Drone: A Deep Dive into the Technology
2025-06-30